Reactions of Monosaccharides
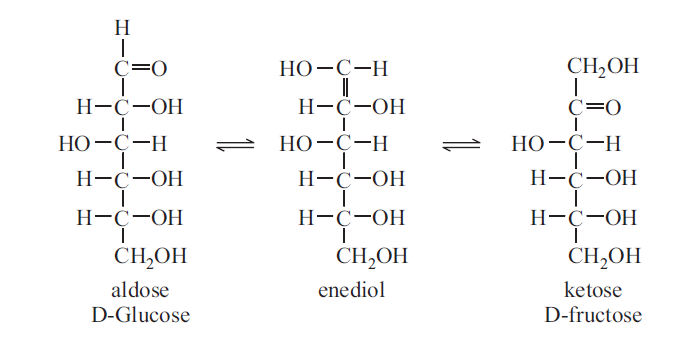
Enediol formation
In mild alkaline solutions, carbohydrates containing free sugar group (aldehyde or keto group) will tautomerise to form enediols, where two hydroxyl groups are attached to the double-bonded carbon, in mild alkaline conditions, glucose is converted into fructose and mannose.
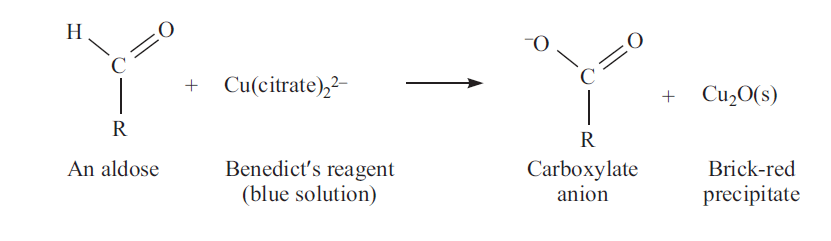
Benedict’s Reaction
Benedict’s reagent contains sodium carbonate, copper sulphate and sodium citrate. In alkaline medium, sugars form enediols which will reduce cupric ions and correspondingly the sugar is oxidized. Any sugar with free aldehyde or keto group will reduce benedict’s reagent and called as reducing sugars.
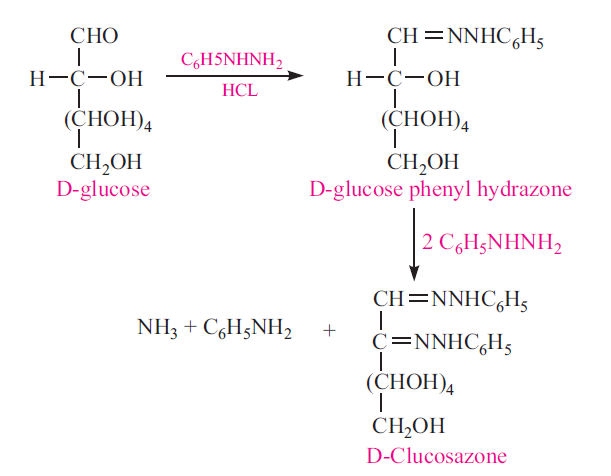
Osazone Formation
All reducing sugars will form osazone with excess of phenylhydrazine when kept at boiling temperature. Osazones are insoluable. Each sugar will have characteristic crystal form of osazone
Oxidation of Sugars
Under mild oxidation conditions the aldehyde group is oxidized to carboxyl group to produce aldonic acid. Ex: Glucose to Gluconic acid When aldehyde group is protected and the molecule is oxidized the last carbon becomes COOH group to produce uronic acid. Ex: Glucose to Glucuronic acid.
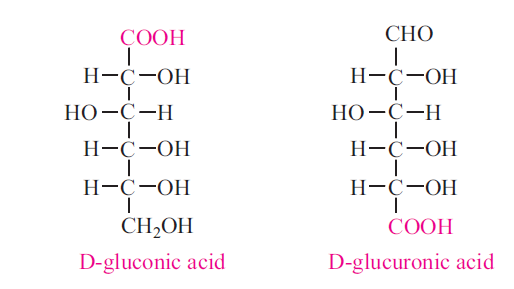
Under strong oxidation conditions the first and last carbon atoms are simultaneously oxidized to form dicarboxyllic acids, known as saccharic acids. Ex: Glucose to Glucosaccharic acid.
Furfural Derivatives
Monosaccharides when treated with concentrated sulphuric acid undergo dehydration with removal of 3 molecules of water. Therefore hexoses give hydroxymethyl furfural and pentoses give furfural. These furfural derivatives can condense with phenolic compounds to give coloured products. This forms the basis of Molisch test, a general test for carbohydrates.
Reduction to form Alcohols
When treated with reducing agents such as sodium amalgam, hydrogen can reduce sugars. Aldose yields corresponding alcohols. But ketose forms two alcohols, because of appearance of a new asymmetric carbon atom. Ex: Glucose forms sorbitol and fructose forms sorbitol and mannitol.
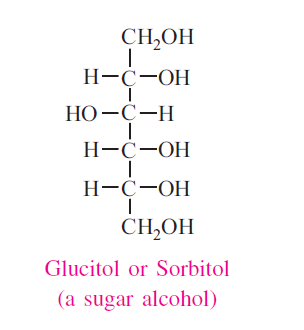
Glycosides: When the hemi-acatal group of a monosaccharide is condensed with an alcohol or phenol group, it is called glycoside.
Formation of esters: Hydroxyl groups of sugars can be esterified to form acetates, propionates, benzoates, phosphates, etc
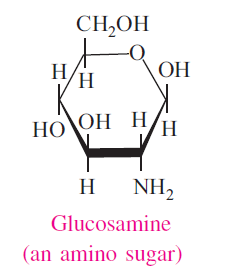
Amino groups may be substituted for hydroxyl groups of sugars to give rise to amino sugars. Ex: Glucose to Glucosamine. Generally the amino group is added to the second carbon atom of hexoses. The amino group may be further acetylated to form N-acetylated sugars such N-acetyl-glucosamine.
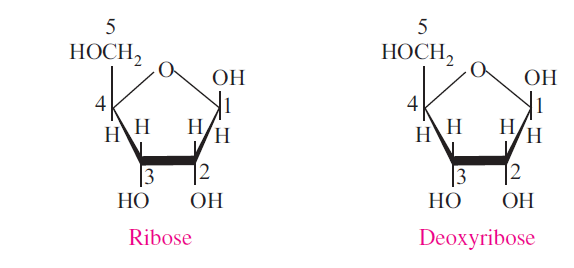
Deoxy sugars: Oxygen of the hydroxyl group may be removed to form deoxy sugars.
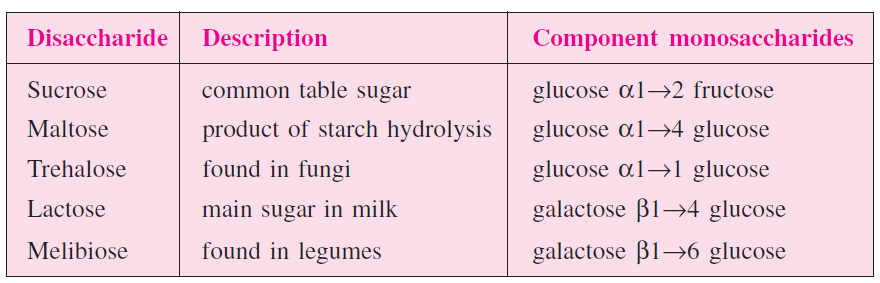
Disaccharides
When two monosaccharides are combined together with elimination of a water molecule it is called disaccharide. Monosaccharides are combined by glycosidic bond.
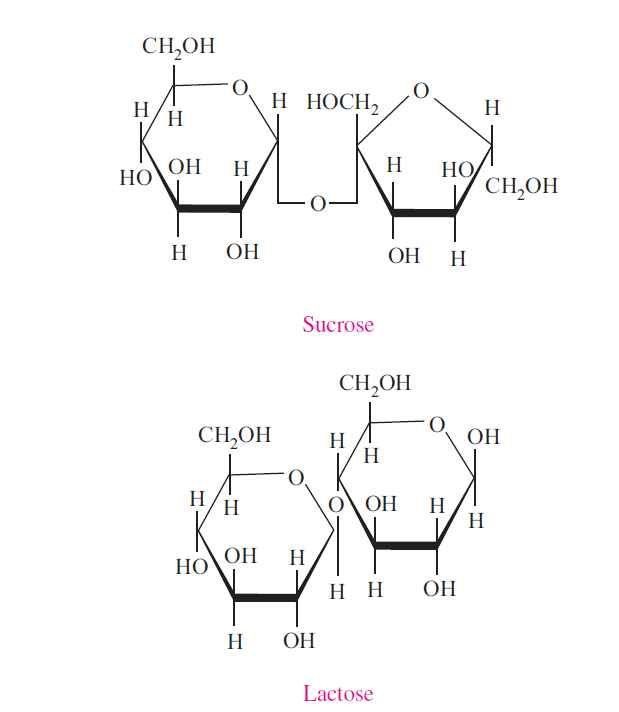
Sucrose
Sucrose also called saccharose, is ordinary table sugar refined from sugar cane or sugar beets. Sucrose is not a reducing sugar. This is because the glycosidic linkage inolves first carbon of glucose and second carbon of fructose, and hence there is no free reducing groups. When sucrose is hydrolyzed the resulting products have reducing property. Hydrolysis of sucrose (optical rotation +66.5°) will produce one molecule of glucose (+52.5°) and one molecule of fructose (- 92°). Therefore the products will change the dextrorotation to levorotation, or the plane of rotation is inverted. Equimolecular mixture of glucose and fructose thus formed is called invert sugar.
Lactose
Lactose is the sugar present in milk. It is reducing disaccharide.
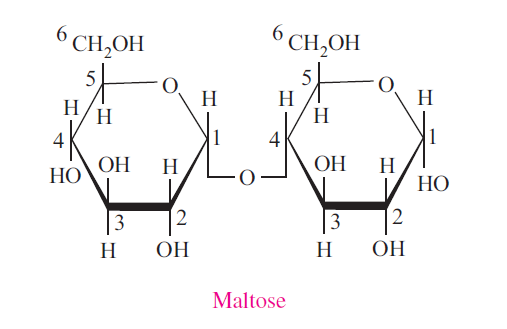
Maltose
Maltose consists of two α-D-glucose molecules. It is a reducing disaccharide
Polysaccharides
Polysaccharides are polymerized products of many monosaccharide units. They may be homo or hetero polysaccharides. Many polysaccharides, unlike sugars, are insoluble in water. Dietary fiber includes polysaccharides and oligosaccharides that are resistant to digestion and absorption in the human small intestine but which are completely or partially fermented by microorganisms in the large intestine.
Homopolysaccharides
They have only one type of monosaccharide units.
Starch
Starch is the major form of stored carbohydrate in plants. Starch is composed of a mixture of two substances:amylose, an essentially linear polysaccharide, and amylopectin, a highly branched polysaccharide. Both forms of starch are polymers of α-D-Glucose. Natural starches contain 10-20% amylose and 80- 90% amylopectin. Amylose forms a colloidal dispersion in hot water (which helps to thicken gravies) whereas amylopectin is completely insoluble.
- Amylose molecules consist typically of 200 to 20,000 glucose units which form a helix as a result of the bond angles between the glucose units.
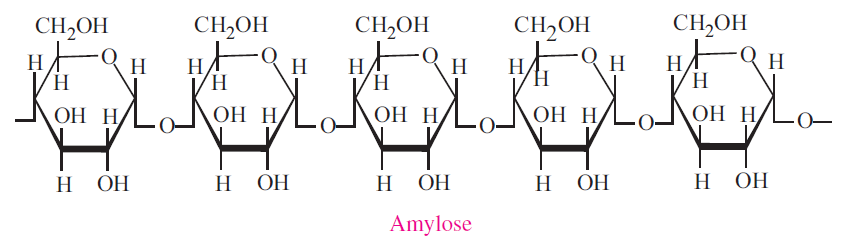
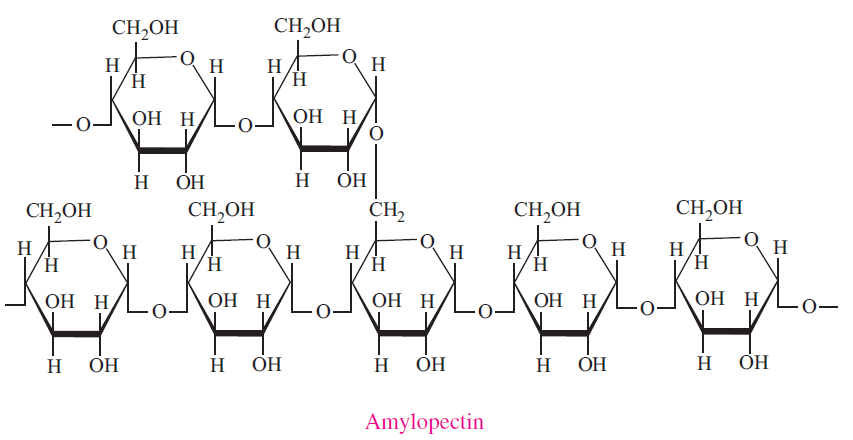
- Amylopectin differs from amylose in being highly branched. Short side chains of about 30 glucose units are attached with 1α→6 linkages approximately every twenty to thirty glucose units along the chain. Amylopectin molecules may contain up to two million glucose units. The side branching chains are clustered together within the amylopectin molecule
Glycogen
Glucose is stored as glycogen in animal tissues by the process of glycogenesis. When glucose cannot be stored as glycogen or used immediately for energy, it is converted to fat. Glycogen is a polymer of α-D-Glucose identical to amylopectin, but the branches in glycogen tend to be shorter (about 13 glucose units) and more frequent. The glucose chains are organized globularly like branches of a tree originating from a pair of molecules of glycogenin, a protein with a molecular weight of 38,000 that acts as a primer at the core of the structure. Glycogen is easily converted back to glucose to provide energy.

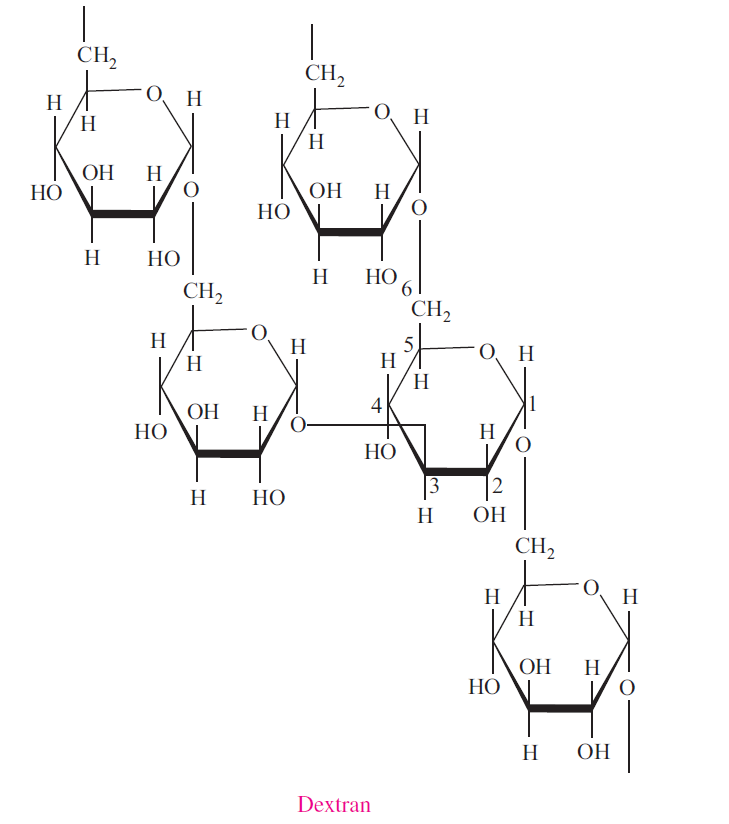
Dextran
Dextran is a polysaccharide similar to amylopectin, but the main chains are formed by 1α→6 glycosidic linkages and the side branches are attached by 1α→3 or 1α→4 linkages. Dextran is an oral bacterial product that adheres to the teeth, creating a film called plaque. It is also used commercially in confections, in lacquers, as food additives, and as plasma volume expanders.
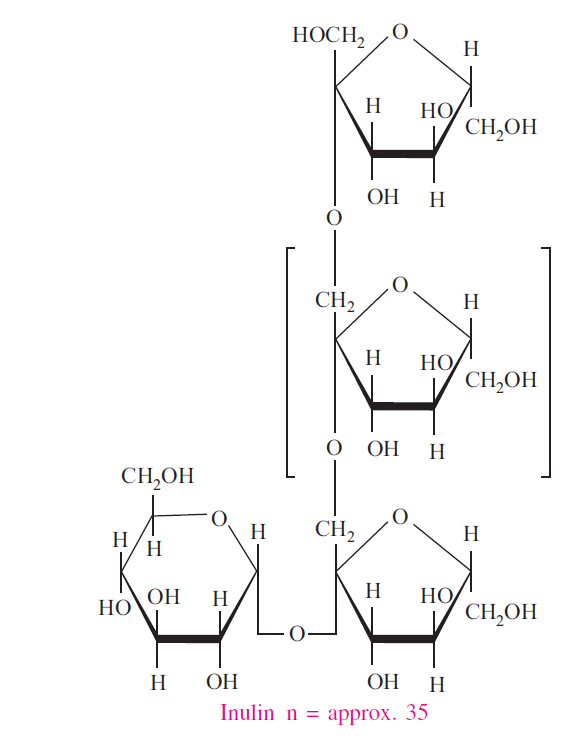
Inulin: Inulins, also called fructans, are polymers consisting of fructose units that typically have a terminal glucose. Oligofructose has the same structure as inulin, but the chains consist of 10 or fewer fructose units. Oligofructose has approximately 30 to 50 percent of the sweetness of table sugar. Inulin is less soluble than oligofructose and has a smooth creamy texture that provides a fatlike mouthfeel. Inulin and oligofructose are nondigestible by human intestinal enzymes, but they are totally fermented by colonic microflora. The short-chain fatty acids and lactate produced by fermentation contribute 1.5 kcal per gram of inulin or oligofructose. Inulin and oligofructose are used to replace fat or sugar and reduce the calories of foods like ice cream, dairy products, confections and baked goods.
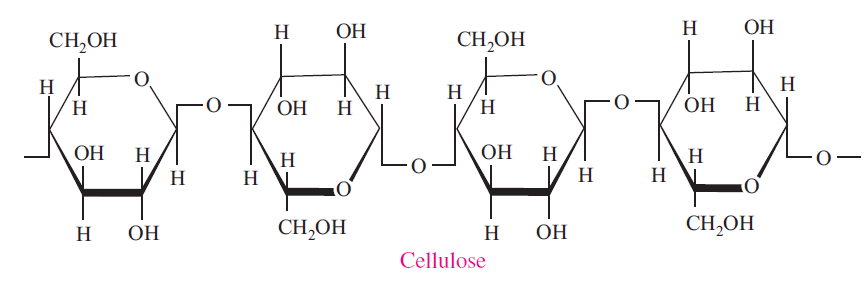
Cellulose
Cellulose is a polymer of â-D-Glucose, which in contrast to starch, is oriented with –CH2OH groups alternating above and below the plane of the cellulose molecule thus producing long, unbranched chains. The absence of side chains allows cellulose molecules to lie close together and form rigid structures. Cellulose is the major structural material of plants. Wood is largely cellulose, and cotton is almost pure cellulose. Cellulose can be hydrolyzed to its constituent glucose units by microorganisms that inhabit the digestive tract of termites and ruminants. Cellulose may be modified in the laboratory by treating it with nitric acid (HNO3) to replace all the hydroxyl groups with nitrate groups (-ONO2) to produce cellulose nitrate (nitrocellulose or guncotton) which is an explosive component of smokeless powder. Partially nitrated cellulose, known as pyroxylin, is used in the manufacture of collodion, plastics, lacquers, and nail polish.
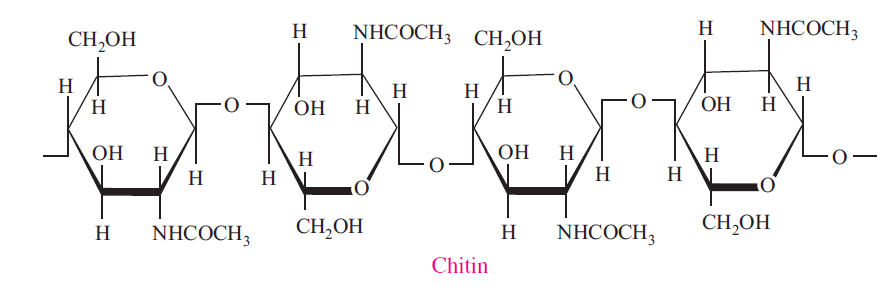
Chitin
Chitin is an unbranched polymer of N-Acetyl-D-glucosamine. It is found in fungi and is the principal component of arthropod and lower animal exoskeletons, e.g., insect, crab, and shrimp shells. It may be regarded as a derivative of cellulose, in which the hydroxyl groups of the second carbon of each glucose unit have been replaced with acetamido (–NH(C=O)CH3) groups.