Sterioisomers
Compounds having same structural formula but differing in spatial configuration are known as sterioisomers. While writing the molecular formula of monosaccharides, the spatial arrangements of H and OH groups are important, since they contain asymmetric carbon atoms. Asymmetric carbon means four different groups are attached to the same carbon. The reference molecule is glyceraldehyde which has a single asymmetric carbon.
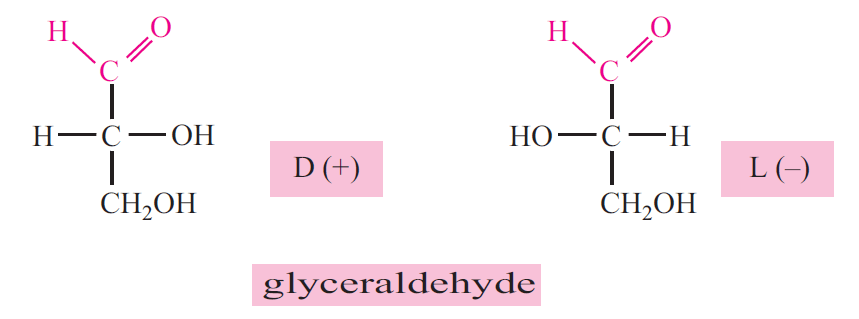
The number of possible sterioisomers depends on the number of asymmetric carbon atoms by the formula 2n where n is the number of asymmetric carbon atoms
Reference carbon atom of sugars
The configuration of H and OH at the second carbon of glyceraldehyde will form two mirror images, they are denoted as D and L varieties. All monosaccharides are molecules derived from glyceraldehyde by successive addition of carbon atoms. Therefore the penultimate carbon atom is the reference carbon atom for naming the mirror images. This is also referred to as absolute configuration.
Optical activity
The presence of asymmetrical carbon atom causes optical activity. When a beam of plane polarized light is passed through a solution of carbohydrates, it will rotate the light either to right or to left. Depending on the rotation, molecules are called dextrorotatory (+) (d) or levorotatory (–).
Racemic mixture
Equimolecular mixture of optical isomers has no net rotation and hence it is called as racemic mixture.
Epimers
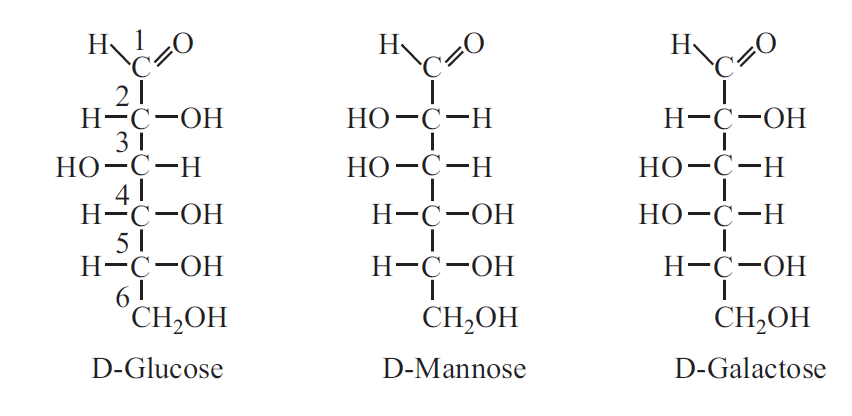
When sugars are different from one another, only in configuration with regard to the single carbon atom, other than the reference carbon atom, they are called Epimers. Example Glucose and Mannose are an epimeric pair which differ only with respect to C2. Similarly galactose is the fourth epimer of glucose
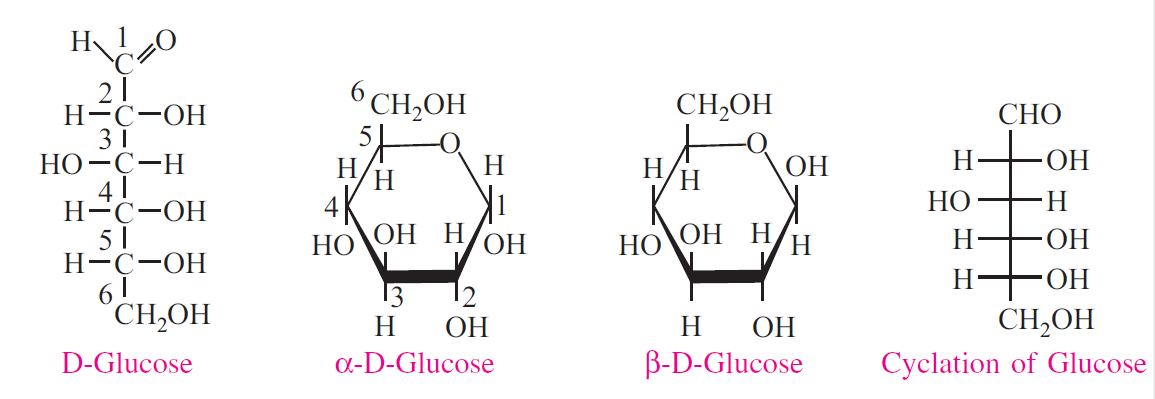
Anomers
When D glucose is crystallized at room temperature and a fresh solution is prepared, its specific rotation of polarized light is +112°; but after 12-18 hours it changes to +52.5°. If initial crystallization is taking place at 98° C and then solubilized, the specific rotation is found to be +19°, which also changes to+52.5° within few hours. This change in rotation with time is called mutarotation. This is explained by the fact that D-glucose has two anomers, alpha and beta varieties. These anomers are produced by the spatial configuration with reference to the first carbon atom in aldoses and second carbon in ketoses. Hence
these carbons are called anomeric carbon atoms. Thus α-D-glucose has specific rotation of +112° and β=D-glucose has specific rotation of +19°. Both undergo mutarotation and at equilibrium one-third molecules are alpha type and two-third are beta variety to get the specific rotation +52.5°