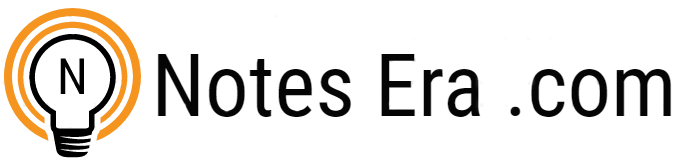Amino Acids are Chiral Molecules
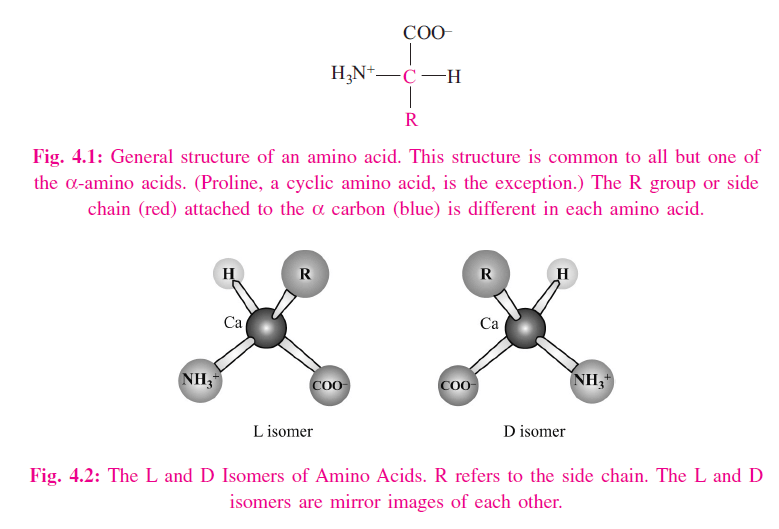
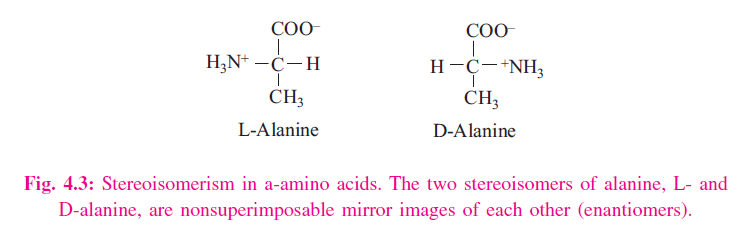
An a-amino acid consists of a central carbon atom, called the a carbon, linked to an amino group, a carboxylic acid group, a hydrogen atom, and a distinctive R group. For all the common amino acids except glycine, the a carbon is bonded to four different groups: a carboxyl group, an amino group, an R group, and a hydrogen atom (Fig. 4.1) in glycine, the R group is another hydrogen atom). The a-carbon atom is thus a chiral center. Because of the tetrahedral arrangement of the bonding orbitals around the a-carbon atom, the four different groups can occupy two unique spatial arrangements, and thus amino acids have two possible stereoisomers. Since they are nonsuperimposable mirror images of each other (Fig. 4.2), the two forms represent a class of stereoisomers called enantiomers (Fig. 4.3). The R group is often referred to as the side chain. Enantiomeric molecules display a special property called optical activity – the ability to rotate the plane of polarization of plane-polarized light. Clockwise rotation of incident light is referred to as dextrorotatory (D) behavior, and counterclockwise rotation is called levorotatory (L) behavior. Only L amino acids are constituents of proteins. The magnitude and direction of the optical rotation depend on the nature of the amino acid side chain.