The Respiratory Chain And Oxidative Phosphorylation
Aerobic organisms are able to capture a far greater proportion of the available free energy of respiratory substrates than anaerobic organisms. Most of this takes place inside mitochondria, which have been termed the “powerhouses” of the cell. Respiration is coupled to the generation of the high-energy intermediate, ATP, by oxidative phosphorylation, and the chemiosmotic theory offers insight into how this is accomplished. A number of drugs (eg, amobarbital) and poisons (eg, cyanide, carbon monoxide) inhibit oxidative phosphorylation, usually with fatal consequences. Several inherited defects of mitochondria involving components of the respiratory chain and oxidative phosphorylation have been reported. Patients present with myopathy and encephalopathy and often have lactic acidosis.
Specific enzymes act as markers
Mitochondria have an outer membrane that is permeable to most metabolites, an inner membrane that is selectively permeable, and a matrix within. The outer membrane is characterized by the presence of various enzymes, including acyl- CoA synthetase and glycerolphosphate acyltransferase. Adenylyl kinase and creatine kinase are found in the intermembrane space. The phospholipid cardiolipin is concentrated in the inner membrane together with the enzymes of the respiratory chain.
The respiratory chain collects and oxidizes reducing equivalents
Most of the energy liberated during the oxidation of carbohydrate, fatty acids, and amino acids is made available within mitochondria as reducing equivalents (H+ or electrons). Mitochondria contain the respiratory chain, which collects and
transports reducing equivalents directing them to their final reaction with oxygen to form water, the machinery for trapping the liberated free energy as high-energy phosphate, and the enzymes of ß-oxidation and of the citric acid cycle that
produce most of the reducing equivalents.
Components of the respiratory chain are arranged in order of increasing redox potential
The respiratory chain consists of a number of redox carriers that proceed from the NAD-linked dehydrogenase systems, through flavoproteins and cytochromes, to molecular oxygen. Not all substrates are linked to the respiratory chain through NAD-specific dehydrogenases; some, because their redox potentials are more positive (eg, fumarate/succinate; are linked directly to flavoprotein dehydrogenases, which in turn are linked to the cytochromes of the respiratorychain.
Ubiquinone or Q (coenzyme Q)
Coenzyme Q links the flavoproteins to cytochrome b, the member of the cytochrome chain of lowest redox potential. Q exists in the oxidized quinone or reduced quinol form under aerobic or anaerobic conditions, respectively. The
structure of Q is very similar to that of vitamin K and vitamin E and of plastoquinone, found in chloroplasts. Q acts as a mobile component of the respiratory chain that collects reducing equivalents from the more fixed flavoprotein complexes and passes them on to the cytochromes. An additional component is the iron-sulfur protein (FeS; nonheme iron). It is associated with
the flavoproteins (metalloflavoproteins) and with cytochrome b. The sulfur and iron are thought to take part in the oxidoreduction mechanism between flavin and Q, which involves only a single e-change, the iron atom undergoing
oxidoreduction between Fe2+ and Fe3+.
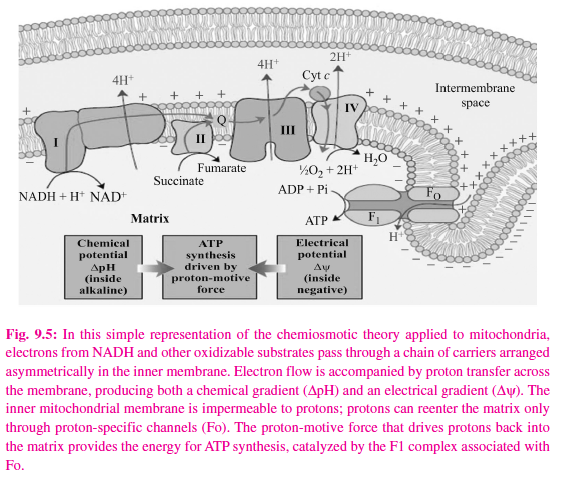
Pyruvate and a-ketoglutarate dehydrogenase have complex systems involving lipoate and FAD prior to the passage of electrons to NAD, while electron trans fers from other dehydrogenases, e.g., L(+)-3-hydroxyacyl- CoA dehydrogenase, couple directly with NAD. The reduced NADH of the respiratory chain is in turn oxidized by a metalloflavoprotein enzyme – NADH dehydrogenase. This enzyme contains FeS and FMN, is tightly bound to the respiratory chain, and passes reducing equivalents on to Q. Electrons flow from Q through the series of cytochromes in order of increasing redox potential to molecular oxygen. The terminal cytochrome aa3 (cytochrome oxidase), responsible for the final combination of reducing equivalents with molecular oxygen, has a very high affinity for oxygen, allowing the respiratory chain to function at maximum rate until the tissue has become depleted of O2. Since this is an irreversible reaction (the only one in the chain), it gives direction to the movement of reducing equivalents and to the production of ATP, to which it is coupled. Functionally and structurally, the components of the respiratory chain are present in the inner mitochondrial membrane as four protein-lipid respiratory chain complexes that span the membrane. Cytochrome c is the only soluble cytochrome and, together with Q, seems to be a more mobile component of the respiratory chain connecting the fixed complexes. The overall reaction is given in the figure 9.5.
The respiratory chain provides most of the energy captured during catabolism
ADP captures, in the form of high-energy phosphate, a significant proportion of the free energy released by catabolic processes. The resulting ATP has been called the energy “currency” of the cell because it passes on this free energy to drive those processes requiring energy. There is a net direct capture of two highenergy phosphate groups in the glycolytic reactions, equivalent to approximately 103.2 kJ/mol of glucose. (In vivo, ?G for the synthesis of ATP from ADP has been calculated as approximately 51.6 kJ/mol. (It is greater than ?G0′ for the hydrolysis of ATP, which is obtained under standard concentrations of 1.0 mol/ L.) Since 1 mol of glucose yields approximately 2870 kJ on complete combustion, the energy captured by phosphorylation in glycolysis is small. Two more high-energy phosphates per mole of glucose are captured in the citric acid cycle during the conversion of succinyl CoA to succinate. All of these phosphorylations occur at the substrate level. When substrates are oxidized via an NAD-linked dehydrogenase and the respiratory chain, approximately 3 mol of inorganic phosphate are incorporated into 3 mol of ADP to form 3 mol of ATP per half mol of O2 consumed; ie, the P:O ratio = 3. On the other hand, when a substrate is oxidized via a flavoprotein- linked dehydrogenase, only 2 mol of ATP are formed; ie, P:O = 2. These reactions are known as oxidative phosphorylation at the respiratory chain level. Such dehydrogenations plus phosphorylations at the substrate level can now account for 68% of the free energy resulting from the combustion of glucose, captured in the form of high-energy phosphate. It is evident that the respiratory chain is responsible for a large proportion of total ATP formation.
Respiratory control ensures a constant supply of ATP
The rate of respiration of mitochondria can be controlled by the availability of ADP. This is because oxidation and phosphorylation are tightly coupled; ie, oxidation cannot proceed via the respiratory chain without concomitant
phosphorylation of ADP. When work is performed, ATP is converted to ADP, allowing more respiration to occur, which in turn replenishes the store of ATP. Under certain conditions, the concentration of inorganic phosphate can also affect the rate of functioning of the respiratory chain. There is also the possibility that the ADP/ATP transporter, which facilitates entry of cytosolic ADP into and ATP out of the mitochondrion, becomes rate limiting. Thus, the manner in which biologic oxidative processes allow the free energy resulting from the oxidation of foodstuffs to become available and to be captured is stepwise, efficient (approximately 68%), and controlled – rather than explosive, inefficient, and uncontrolled, as in many nonbiologic processes. The remaining free energy that is not captured as high-energy phosphate is liberated as heat. This need not be considered “wasted,” since it ensures that the respiratory system as a whole is sufficiently exergonic to be removed from equilibrium, allowing continuous unidirectional flow and constant provision of ATP. It also contributes to maintenance of body temperature.
Many poisons inhibit the respiratory chain
Much information about the respiratory chain has been obtained by the use of inhibitors, and, conversely, this has provided knowledge about the mechanism of action of several poisons (Figure 9.6). They may be classified as inhibitors of the respiratory chain, inhibitors of oxidative phosphorylation, and uncouplers of oxidative phosphorylation. Barbiturates such as amobarbital inhibit NAD linked dehydrogenases by blocking the transfer from FeS to Q. At sufficient dosage, they are fatal in vivo. Antimycin A and dimercaprol inhibit the respiratory chain between cytochrome b and cytochrome c. The classic poisons H2S, carbon monoxide, and cyanide inhibit cytochrome oxidase and can therefore totally arrest respiration. Malonate is a competitive inhibitor of succinate dehydrogenase. Atractyloside inhibits oxidative phosphorylation by inhibiting the transporter of ADP into and ATP out of the mitochondrion. The action of uncouplers is to dissociate oxidation in the respiratory chain from phosphorylation. These compounds are toxic in vivo, causing respiration to become uncontrolled, since the rate is no longer limited by the concentration of ADP or Pi. The uncoupler that has been used most frequently is 2,4- dinitrophenol, but other compounds act in a similar manner. The antibiotic oligomycin completely blocks oxidation and phosphorylation by acting on a step in phosphorylation.
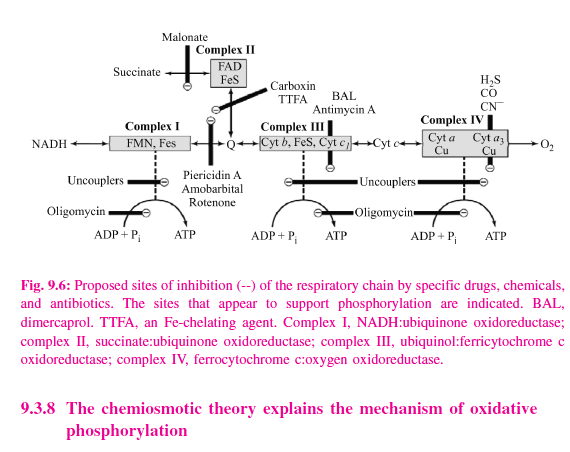
The chemiosmotic theory explains the mechanism of oxidative phosphorylation
Mitchell’s chemiosmotic theory postulates that the energy from oxidation of components in the respiratory chain is coupled to the translocation of hydrogen ions (protons, H+) from the inside to the outside of the inner mitochondrial membrane. The electrochemical potential difference resulting from the asymmetric distribution of the hydrogen ions is used to drive the mechanism responsible for the formation of ATP (Figure 9.7).
The respiratory chain is a proton pump
Each of the respiratory chain complexes I, III, and IV act as a proton pump. The inner membrane is impermeable to ions in general but particularly to protons, which accumulate outside the membrane, creating an electrochemical potential difference across the membrane. This consists of a chemical potential (difference in pH) and an electrical potential.
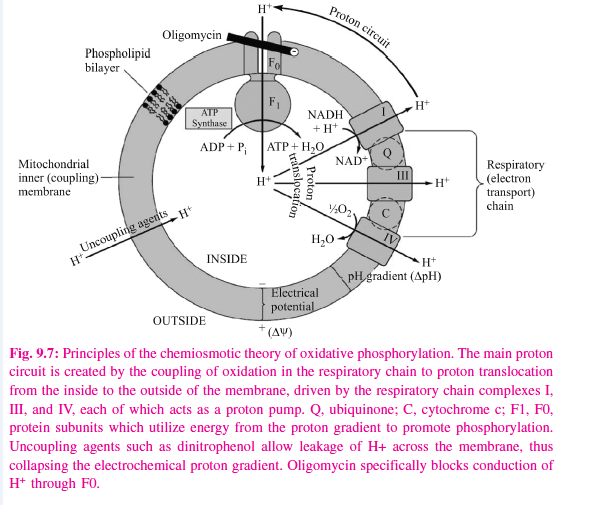
A membrane-located ATP synthase functions as a rotary motor to form ATP
The electrochemical potential difference is used to drive a membrane-located ATP synthase which in the presence of Pi + ADP forms ATP. Scattered over the surface of the inner membrane are the phosphorylating complexes, ATP synthase, responsible for the production of ATP. These consist of several protein subunits, collectively known as F1, which project into the matrix and which contain the phosphorylation mechanism. These subunits are attached to a membrane protein complex known as F0, which also consists of several protein subunits. F0 spans the membrane and forms the proton channel. The flow of protons through F0 causes it to rotate, driving the production of ATP in the F1 complex. Estimates suggest that for each NADH oxidized, complex I translocates four protons and complexes III and IV translocate 6 between them. As four protons are taken into the mitochondrion for each ATP exported, the P:O ratio would not necessarily be a complete integer, ie, 3, but possibly 2.5. However, for simplicity, a value of 3 for the oxidation of NADH + H+ and 2 for the oxidation of FADH2 will continue to be used throughout this text.